Watch these videos in You tube
Molecules in solids Eureka (http://youtu.be/AhBGMdhJ4nA)
Molecules in liquids Eureka(http://youtu.be/hxqEUy9Dusk)
Evaporation and Condensation Eureka(http://youtu.be/yyxc-81JDbo)
Phase changes (http://youtu.be/8mqoxGiuKeA)
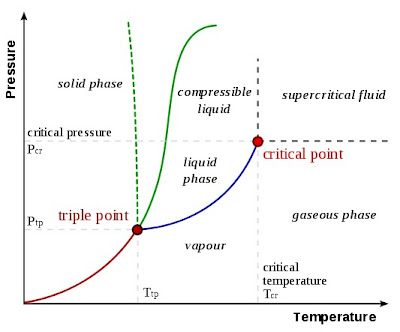
Melting, or fusion, is a physical process that results in the phase change of a substance from a solid to a liquid.
Freezing or solidification is a phase change in which a liquid turns into a solid when its temperature is lowered below its freezing point. The reverse process is melting
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. (The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid)
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding environmental pressure. While below the boiling point a liquid evaporates from its surface, at the boiling point vapor bubbles come from the bulk of the liquid.
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization
Sublimation is the process of transition of a substance from the solid phase to the gas phase without passing through an intermediate liquid phase.
Deposition is a process in which gas transforms into solid (also known as desublimation). The reverse of deposition is sublimation.
SHOW YOUR KNOWLEDGE
Good Videos to understand this topic:
States of matter (http://youtu.be/HAPc6JH85pM)
The Phase Changes of Water Song - Mr. Edmonds (http://youtu.be/8eALQ2HP45I)
States of Matter and Phase Change (http://youtu.be/BjyXIZtlHFo)





No hay comentarios:
Publicar un comentario